Categories Mathematics Leave a Reply Cancel replyThe CtoN and HtoN molar ratios are adequately close to whole numbers, and so the empirical formula is C 5 H 7 N The empirical formula mass for this compound is therefore 8113 amu/formula unit, or 8113 g/mol formula unit Calculate the molar mass for nicotine from the given mass and molar amount of compoundIt represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together The expression 2H, on the other hand, indicates

Solarsect 2 N 1 Insect Repel Services
N(n-1)/2 formula in chemistry
N(n-1)/2 formula in chemistry-Illustrated Glossary of Organic Chemistry n1 rule When splitting is firstorder , the NMR signal for a nucleus having n neighbors is split into n1 lines The 1 HNMR spectrum of 2 methoxy butane , illustrating the n1 rule n 1 and n 2 are integers where n 2 > n 1 It was later found that n 2 and n 1 were related to the principal quantum number or energy quantum number This formula works very well for transitions between energy levels of a hydrogen atom with only one electron



Q Tbn And9gcswjtndnyd2 U4usxcb4q9aftasxekclokt8hytbwohnunqh1gq Usqp Cau
2n 2 1 st energy level has;The formula n (n − 1) / 2 for the number of pairs you can form from an n element set has many derivations, even many on this site One is to imagine a room with n people, each of whom shakes hands with everyone else If you focus on just one person you see that she participates inComplete the recursive formula of the arithmetic sequence 8, 5, 18, 31, d(1) = d(n) = d(n1) Complete the recursive formula of the arithmetic sequence 8, 5, 18, 31, d(1) =
How do you find a formula for the sum n terms #sum_(i=1)^n (1i/n)(2/n)# and then find the limit as #n>oo#?Two comments before showing some examples 1) a 1 in chemistry is usually dropped, so that Na 1 equal Na or Mg 1 Cl 2 equals MgCl 2; The table below represents a linear function f(x) and the equation represents a function g(x) x f(x) −1 −5 0 −1 1 3 g(x) g(x) = 2x − 7 part a write a sentence to compare the slope of the two functions and show the steps you used to determine the slope of f(x) and g(x)
Is 1, according to the convention for an empty product The factorial operation is encountered in many areas of mathematics, notably in combinatorics, algebra, and mathematical analysisIts most basic use counts the possible distinct sequences – the permutations – of n distinct objects there are n!The pi electron count is defined by the series of numbers generated from 4n2 where n = zero or any positive integer (ie, n = 0, 1, 2, etc) The most common case in six pi electrons (n = 1) which is found for example in benzene, pyrrole, furan, and pyridine The total number of ways in which it is possible to make groups by taking some or all out of n = (n 1 n 2 ) things, when n 1 are alike of one kind, n 2 are alike of second kind, and so on is {(n 1 1)(n 2 1)} – 1
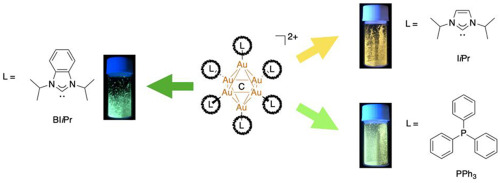



Ligand Effects On The Photophysical Properties Of N N Diisopropylbenzimidazolylidene Protected C Centered Hexagold I Clusters Journal Of Organometallic Chemistry X Mol




Chem Test 3 Own Review Scrap Quantities In Chemical Equations Unit Test Studocu
For example, H 2 and 2H represent distinctly different species H 2 is a molecular formula;Johan Rydberg use Balmers work to derived an equation for all electron transitions in a hydrogen atom Here is the equation R= Rydberg Constant x10 7 m 1;The energy of the first orbit (n = 1) is −136 eV For n = 2, 3, −340 eV, −151 eV Although the energy increases with n, its absolute value decreases An electron in the hydrogen atom is mostly found in its ground state, the lowest energy state The electron moves to a higher energy state, an excited state, when it absorbs energy



Guides Lib Purdue Edu C Php G P




Structural And Spectroscopic Characterization Of A Cationic Aquanitrato Copper Ii Complex With The Tetradentate Schiff Base Ligand N N Bis 1 2 Pyridyl Ethylidene Ethane 1 2 Diamine Pdf Document
The more general formula for this is 2nI 1, where I is the magnetic spin number of the given nucleus And since it is equal to one for hydrogen, the formula that we use in 1 H NMR is n 1 Below is a summary table for the splitting patterns in NMR spectroscopy When two protons split each other's NMR signals, they are said to be coupled Explanation S = n(n 1) 2 S = n2 n 2 2S = n2 n n2 n −2S = 0 using the quadratic formula for ax2 bx c = 0, n = −b ± √b2 −4ac 2a1 λ = k hc(1 n2 1 − 1 n2 2) 1 λ = k h c (1 n 1 2 − 1 n 2 2) which is identical to the Rydberg equation for R∞ = k hc R ∞ = k h c When Bohr calculated his theoretical value for the Rydberg constant, R∞ R ∞, and compared it with the experimentally accepted value, he got excellent agreement
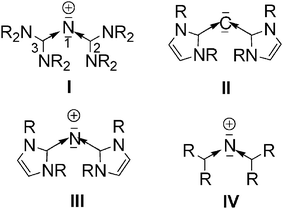



Novel N L 2 Species With Two Lone Pairs On Nitrogen Systems Isoelectronic To Carbodicarbenes Chemical Communications Rsc Publishing




Final Exam With Resolution Honors General Chemistry Chem 155 Docsity
The Principal Quantum Number (\(n\)) The principal quantum number, \(n\), designates the principal electron shell Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom isn can be any positive integer starting at 1, as \(n=1 `omega` `2(1w)(1w^2)3(2w)(2w^2)(n1)(nw)(nw^2)` एक अज्ञात प्रतिरोध प्रतिरोध के श्रेणी क्रम में जोड़ा गया है। इस संयोजन को मीटर सेतु के पहले रिक्त स्थान में जोड़ा गया है जबकिMolecular Weight Date s Modify Create N (1naphthyl)ethylenediamine is an Nsubstituted ethylenediamine compound having 1naphthyl as




Ma Am In This Question Why Can T We Use N N 1 2 Formula To Find The No Of Lines In Chemistry Meritnation Com




Solved The Energy Of The H Atom S Single Electron When B Chegg Com
Solution Proof by induction Base case for 2 nodes there is 1 connection and 2 * 1 / 2 == 1 Now assuming that for N nodes we have N * (N1) / 2 connections Adding one more node has to establish N additional connections, and N * (N 1) / 2 N = (N^ 2 N 2N) / 2 = (N^ 2 N) / 2 = (N 1) * N / 2 This last line is exactly N * (N 1In order to find the normality, we will apply the given formula N = Molarity (M) × number of equivalents N = 10 × 2 (replacing the values) Therefore, normality of the solution = Question 2 Calculate the normality of the solution obtained by dissolving 0321 g of the salt sodium carbonate (Na2CO3) in 250 mL waterThe equation also shows us that as the electron's energy increases (as n increases), the electron is found at greater distances from the nucleus This is implied by the inverse dependence on r in the Coulomb potential, since, as the electron moves away from the nucleus, the electrostatic attraction between it and the nucleus decreases, and it is held less tightly in the atom




Identify Atoms In Chemical Equation Tex Latex Stack Exchange



Q Tbn And9gcswjtndnyd2 U4usxcb4q9aftasxekclokt8hytbwohnunqh1gq Usqp Cau
👍 Correct answer to the question Identify the first 4 terms in the geometric sequence given by the explicit formula ƒ(n) = 4 × 2(n – 1) eeduanswerscomWavelength of light required to excite an electron in a hydrogen atom from level n =1 to n=2 will be (h=662 x 10 34 Js and c = 30 x 10 8 ms 1) 1214 x 10 7 m 2816 x 10 7 m 6500 x 10 7 mIt is important to note that a subscript following a symbol and a number in front of a symbol do not represent the same thing;



1




Chemcomp H1v N N Ethane 1 2 Diylbis Oxyethane 2 1 Diyl Bis 2 6s 4 4 Chl Yorodumi
Formula for the sum 1 2 2 2 3 2 n 4 n (n 1) (2 n 1) − 2 n (n 1) n = 1 4 n 4 n (n 1)The factorial function can also be extended to noninteger argumentsPosted on by gecmisten The formula an=4⋅(2)n−1 represents a sequence If n≥1, what are the first three terms in the sequence?




Silicicacid Is The General Name For A Family Of Chemical Compounds Containing The Element Silicon Attached To Oxide And Hydroxyl Groups This Family Of Compou
:max_bytes(150000):strip_icc()/what-is-the-rydberg-formula-604285_final-251d1441e24e44c88aab687409554ed4.png)



What Is The Rydberg Formula And How Does It Work
2) Orbitals are combined when bonds form between atoms in a molecule There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental) Within each shell of an atom there are some combinations of orbitals In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p The way the items are ordered now you can see that each of those pairs is equal to N (N11 is N, N22 is N) Since there are N1 items, there are (N1)/2 such pairs So you're adding N (N1)/2 times, so the total value is N* (N1)/2The formula an=4⋅(2)n−1 represents a sequence If n≥1, what are the first three terms in the sequence?



Formula Used In Volumetric Analysis Chemical Stoichiometry Chemistry Study Material Emedicalprep Com Emedicalprep




Consider The Equation For The Bohr Model Of The Hydrogen Atom You Graphed E Course Hero
I have wondered how the closed form for the sum of squares for the first n natural numbers was derived Given the formula for the sum 1^22^2n^2= n(n1)(2n1)/6 I learned to prove its correctness using mathematical induction However, I neverN is equal to the energy level (initial and final) If we wanted to calculate energy we can adjust R by multipling by h (planks constant) and c (speed of light)The value of 0!




Table 1 From Synthesis And Structure Of A Binuclear Cu Ii Complex Of 1 3 Bis N N Bis 2 Picolyl Amino Propan 2 Ol Semantic Scholar




Tables Of Math Physics And Chemistry Engineering Handbook
Alkanes are organic compounds that consist of singlebonded carbon and hydrogen atoms The formula for Alkanes is C n H 2n2, subdivided into three groups – chain alkanes, cycloalkanes, and the branched alkanes Alkanes are comprised of a series of compounds that contain carbon and hydrogen atoms with single covalent bondsCalculus Introduction to Integration Sigma Notation 1 AnswerUsing the Bohr model, determine the energy in joules of the photon produced when an electron in a Li 2 ion moves from the orbit with n = 2 to the orbit with n = 1 Consider a large number of hydrogen atoms with electrons randomly distributed in the n = 1, 2, 3, and 4 orbits




4 N 5 Bromofuran 2 Yl Methyl 1 N 1 N Diethylbenzene 1 4 Diamine Formula C15h19brn2o Over 100 Million Chemical Compounds Mol Instincts




Topics On Chemical Kinetics And Quantum Theory Physical Chemistry Ii Ch 342 Docsity
Re Rydberg Formula Postby JasmineAlberto4J » Mon 543 am if you use the equation from the book ν= R n1 will equal your final energy level and n2 will equal your initial energy level So if you transition from n=4 to n=2, n=2 is your final energy level ( 2=n1) and n=4 is your initial energy level (4=n1)2n 2 =21 2 =2 electrons 2 nd energy level has;Maximum number of electrons that can be accommodated in a shell is given by 2n 2 where n = shell number For 1 st energy level, n = 1 Maximum number of electrons in 1 st energy level = 2n 2 2 x (1) 2 = 2 For 2nd energy level n=2 Maximum number of electrons in the 2nd energy level = 2n 2 2 x 22 = 2 x 4 = 8 For 3 rd energy level n=3



Indoaniline Dye 1 Hydroxy 2 2 4 6 Trimethyl Naphthanilide With 4 Amino N N Diethyl 3 Methylaniline




Experimental Details Irans Crystal Data Chemical Formula Pdch N Hs 2 I Download Table
21 For the proof, we will count the number of dots in T (n) but, instead of summing the numbers 1, 2, 3, etc up to n we will find the total using only one multiplication and one division!And 2) since every solid ionic compound is composed of many, many cations and anions, we say that the chemical formula of an ionic compound represents the lowest whole number ratio of elements making up theChemistry Sign up Log in Excel in math and science Formula for the sum 1 2 3 ⋯ n 1 2 3 \cdots n 1 2 3 ⋯ n;




Thiocarbamic Acid N N Dimethyl S 1 3 Diphenyl 2 Butenyl Ester Formula C19h21nos Over 100 Million Chemical Compounds Mol Instincts




Pdf Elemental Chemistry For Moscovium Element 115 Anthony P Bermanseder Academia Edu
λ is the wavelength; Yes n1 is the initial energy level and n2 is the final energy level Both n1 and n2 are integers (1, 2, 3, 4) and n2>n1It would be nice to have some results like Faulhaber's formula, but unfortunately for this problem we don't have a formula like that However, this was a proposed problem in the MAA Journal long long back and the conclusion was that the best one c




The Chemical Structure And Formula Of Acetamiprid A And Download Scientific Diagram




3 Ways To Calculate Bond Order In Chemistry Wikihow
Noncyclic (or acyclic) alkanes have the general formula $\ce{C_{n}H_{2n 2}}$, and corresponding alkenes have the general formula $\ce{C_{n}H_{2n}}$ So in this problem let's say our alkane (x) is $\ce{C_{n}H_{2n 2}}$, then our alkene (y) with one more carbon atom will be $\ce{C_{n 1}H_{2(n 1)}}$ While reading through Narendra Avasthi's Problems in Physical Chemistry, I came across two formulas on p 64 $\begingroup$ the formula gives the total number of transitions provided the cascade of all Thus, total of $1 \times 6 = n_1(n_2n_1)$ (foot note 1) spectral lines would be present in the spectrum Similarly, when therePhysical Chemistry the value of (n2n1)and (n2 2 n1 2 ) for he the value of (n2n1)and (n2 2 n1 2 ) for he ion in atomic spectrum are 4and 8 respectivelythe wavelength of emitted photon when electron jump from n2 to n1



2




2s N N Dimethyl 2 1s 1 2 3 4 Tetrahydronaphthalen 1 Ylformamido Propanamide Formula C16h22n2o2 Over 100 Million Chemical Compounds Mol Instincts
Stack Exchange network consists of 177 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers Visit Stack ExchangeSum of n, n², or n³ n n are positive integers Each of these series can be calculated through a closedform formula The case 5050 5050 5050 ∑ k = 1 n k = n ( n 1) 2 ∑ k = 1 n k 2 = n ( n 1) ( 2 n 1) 6 ∑ k = 1 n k 3 = n 2 ( n 1) 2 42n 2 =23 2 =18 electrons Electrons are located energy levels starting from the first energy levels




Kra New Preparation Of 2 2 N Butyl 4 Hydroxy 6 Methyl Pyrimidine 5 Yl N N Dimethylacetamide Google Patents
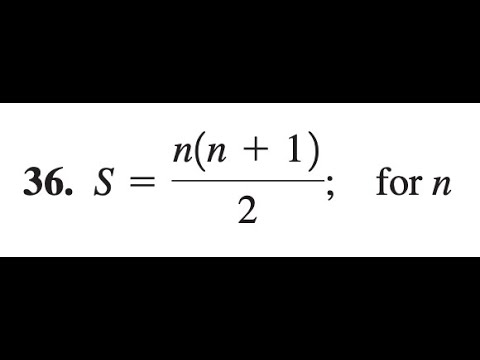



S N N 1 2 Solve For N Youtube
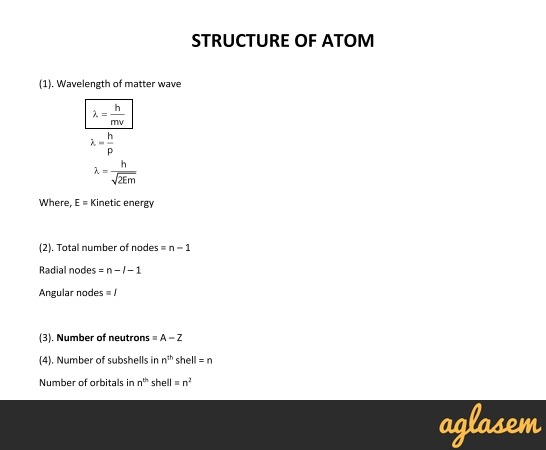
Shells and Subshells of Orbitals Orbitals that have the same value of the principal quantum number form a shellOrbitals within a shell are divided into subshells that have the same value of the angular quantum number Chemists describe the shell and subshell in which an orbital belongs with a twocharacter code such as 2p or 4fThe first character indicates the shell (n = 2 or n = 4)A frequency (or spectral energy) emitted in a transition from n 1 to n 2 therefore represents the photon energy emitted or absorbed when an electron makes a jump from orbital 1 to orbital 2 Later models found that the values for n 1 and n 2 corresponded to the2n 2 =22 2 =8 electrons 3rd energy level has;




Chemical Formulas For Different Biomaterial Based Vectors A Commonly Download Scientific Diagram




Stirling Theorem
E n = − k Z 2 n 2 E n = − k Z 2 n 2 The sizes of the circular orbits for hydrogenlike atoms are given in terms of their radii by the following expression, in which a 0 a 0 is a constant called the Bohr radius, with a value of 5292 × × 10 −11 mTo do this, we will fit two copies of a triangle of dots together, one red and an upsidedown copy in green Eg T (4)=1234For example, we can find number of electrons in four energy level with following formula;
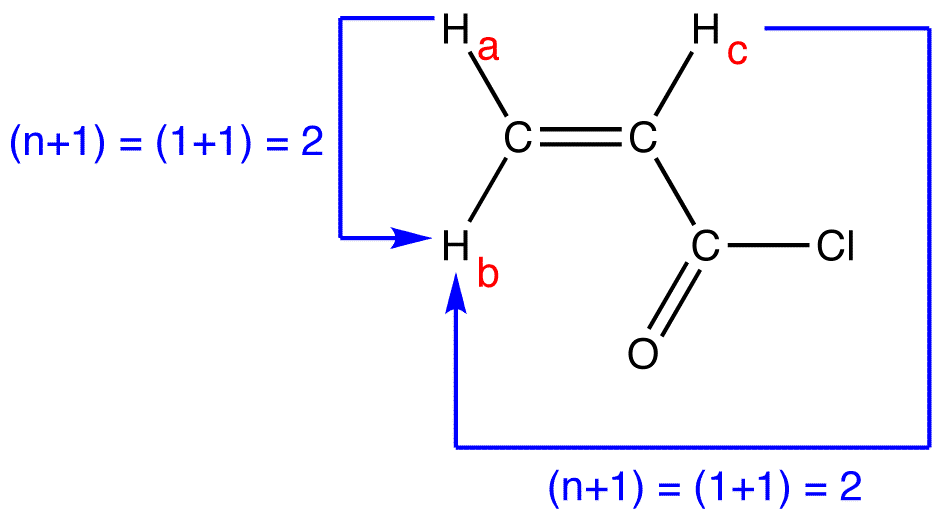



N 1 Rule Chemistry Libretexts




Kra Organic Electroluminescence Device Google Patents




The Equation Alpha D D N 1 D Is Not Correctly Matched For



Chapter Twentyfour Organic Chemistry Chapter Twentyfour Organic Chemistry




4 S Amino 2h 1 2 3 4 Tetrazol 5 Yl Methyl N N Dimethylaniline Formula C10h14n6 Over 100 Million Chemical Compounds Mol Instincts




Solarsect 2 N 1 Insect Repel Services




Naming Compounds And Balancing Chemical Equations Using Oxidation Num




By Rene Boyas Names Chemical Formula Is C 22 H 28 N 2 O Its Chemical Name Is N 1 2 Phenethyl 4 Piperidinyl N Phenyl Propanamide Like Heroin Morphine Ppt Download
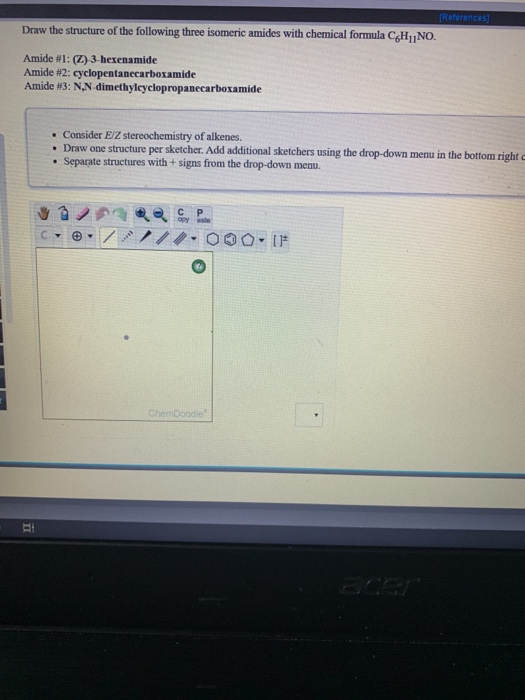



References Draw The Structure Of The Following Three Chegg Com



2




Chemical Formula Of Studied Compounds O 1 Download Scientific Diagram




N N 1 3 Phenylene Bismaleimide Cas 3006 93 7 Haihang Industry
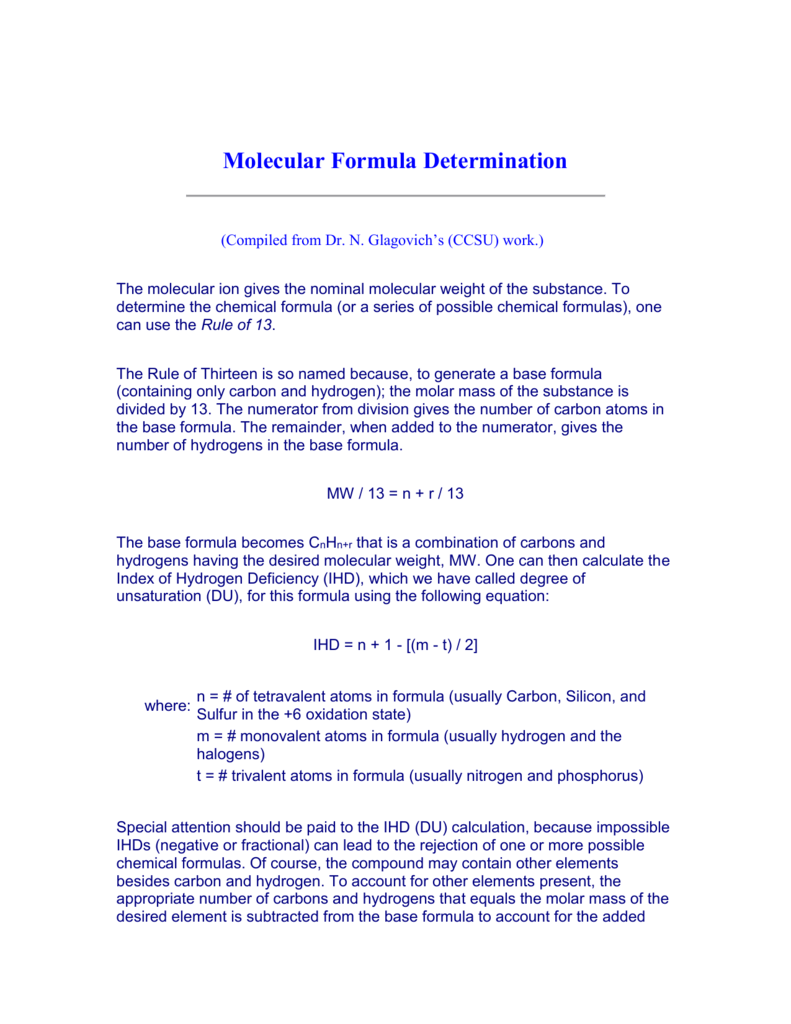



Molecular Formula Determination




Chemical Structures And Synthesis Of Pyrazinacenes A General Formula Download Scientific Diagram




Image Result For Set Theory Cheat Sheet Theoretical Computer Science Math Methods Studying Math
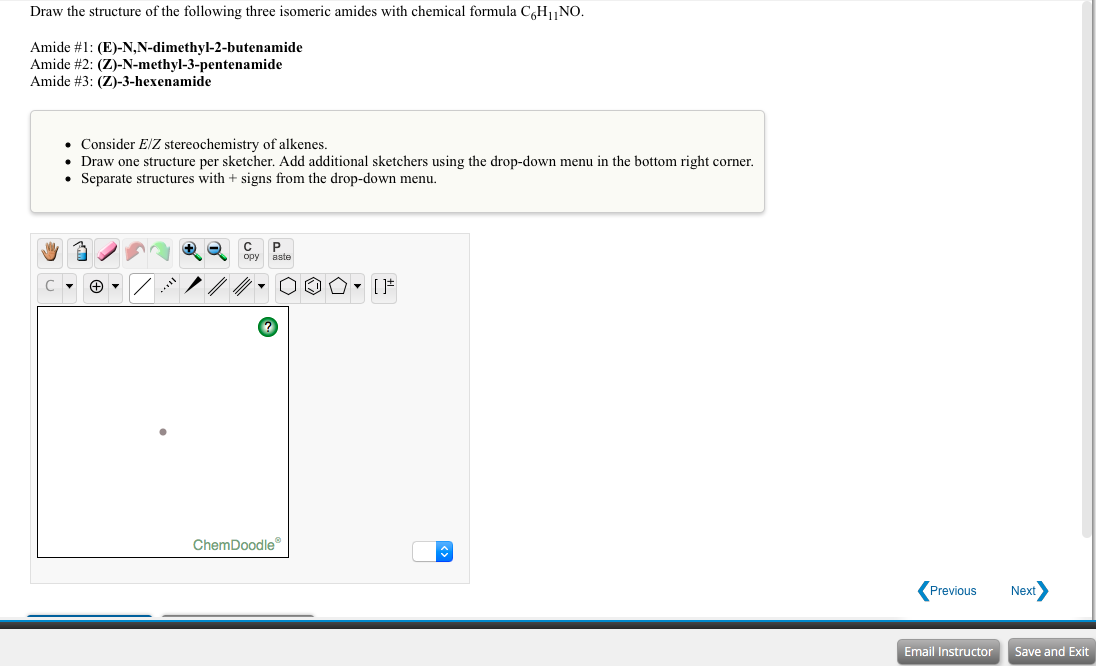



Answered Draw The Structure Of The Following Bartleby



Chemistry Formulas For Class 12 Pdf In Hindi




Chemistry Formulas For Android Apk Download




Pdf Telecharger Chemistry Formula Download Gratuit Pdf Pdfprof Com




Quiz 4 Chemical Rxns And Safety Ppt Powerpoint




N N Dimethyltryptamine Wikipedia




Pdf Extraterrestrial Physics And Elemental Chemistry For Element 115 Moscovium Anthony P Bermanseder Academia Edu




Capb Chemical Structure 1 Propanaminium N Carboxymethyl N Download Scientific Diagram



Q Tbn And9gctom4x3n1mffhzj85qepaggdofhkq4mxrmgujr9al1tacu67bgn Usqp Cau




The Formula For A Hydrate Chemistry 11 5




Chemical Bonding Part 2 Covalent Bonds Chemical Formulas




3 Ways To Calculate Bond Order In Chemistry Wikihow
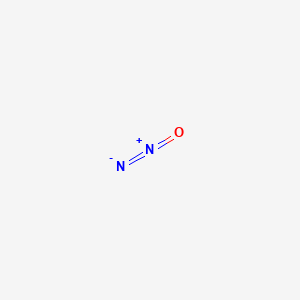



Nitrous Oxide N2o Pubchem
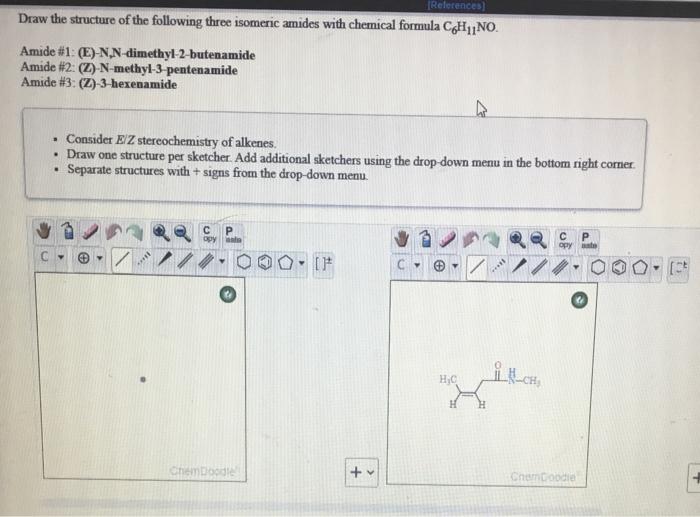



Draw The Structure Of The Following Three Isomeric Chegg Com




N N N Triethyl N 3 Methylphenyl Ethane 1 2 Diamine Formula C15h26n2 Over 100 Million Chemical Compounds Mol Instincts
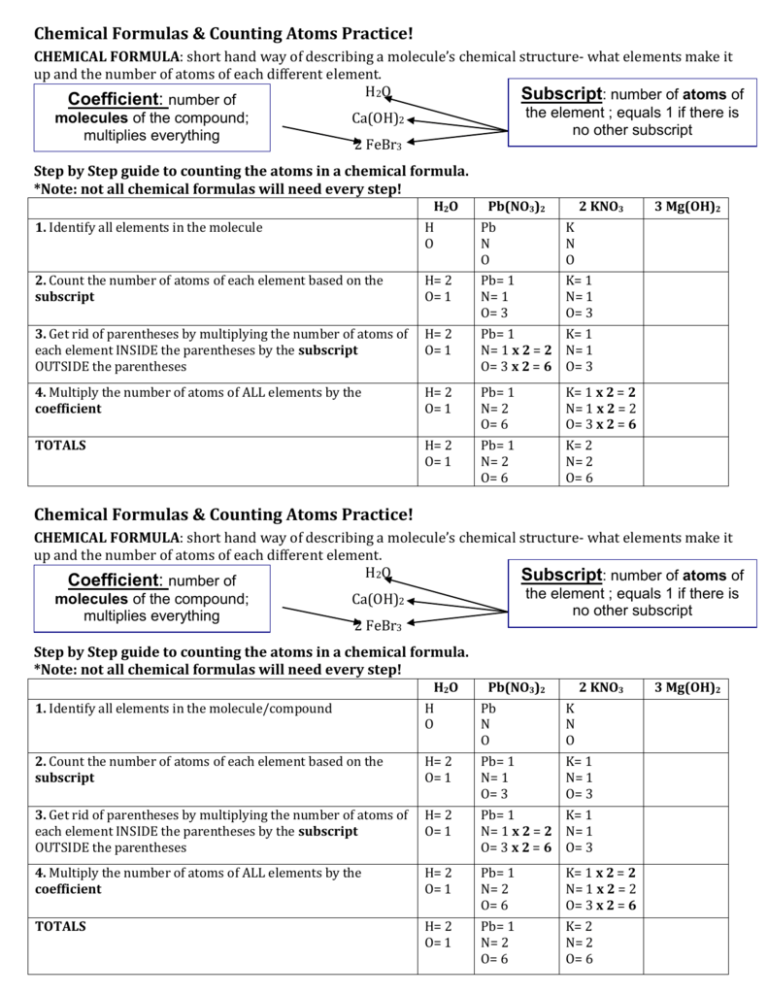



Chemical Formulas Counting Atoms Practice




N N Dimethyl 1 Pentanamine C7h17n Density Melting Point Boiling Point Structural Formula Synthesis
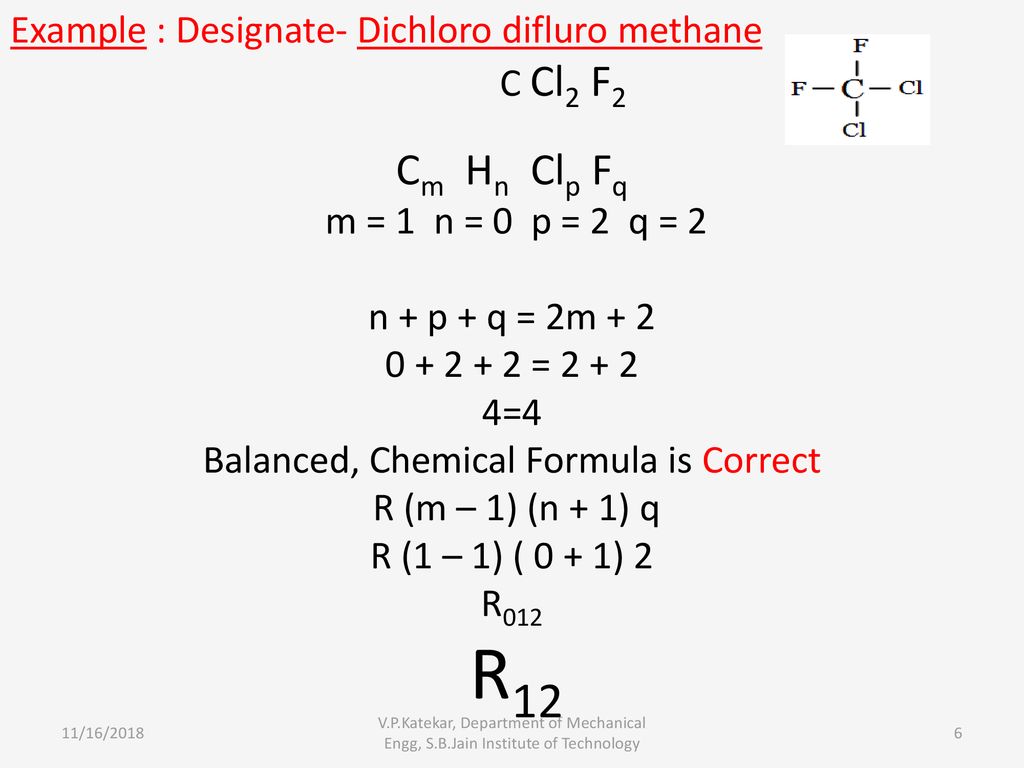



Designation Of Refrigerant Ppt Download




22 554 Chemical Formula Illustrations Clip Art Istock




Balanced Chemical Equation Definition Examples Video Lesson Transcript Study Com




Derive The Formula For The Max No Of Spectral Lines Chemistry Structure Of Atom Meritnation Com




Compounds 1 Types Of Compounds 2 Formula Writing 3 Formula Naming 4 Empirical Formulas 5 Molecular Formulas 6 Types Of Chemical Reactions 7 Balancing Ppt Download




Chemical Formulas More Chemical Engineering Blog Facebook
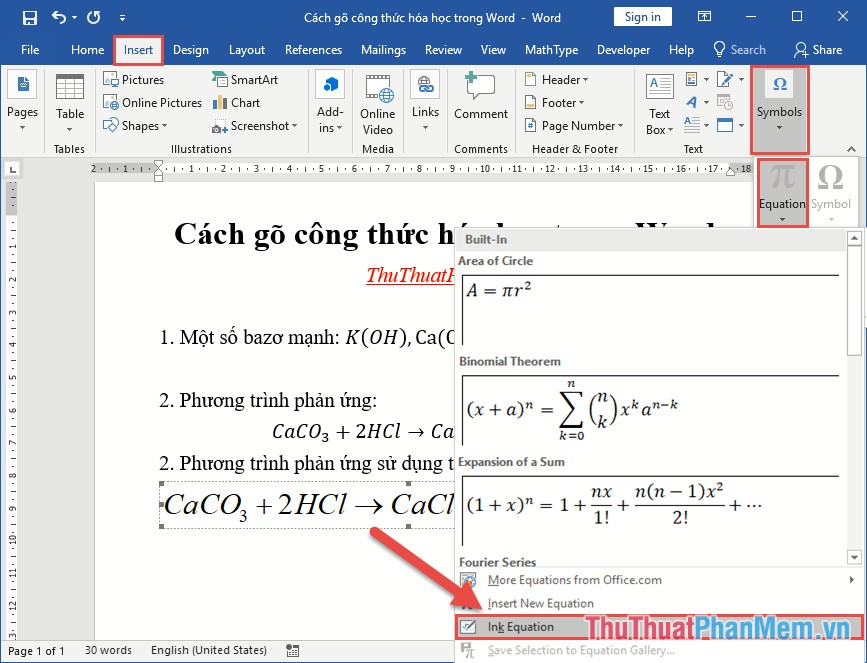



How To Type Chemical Formulas In Word
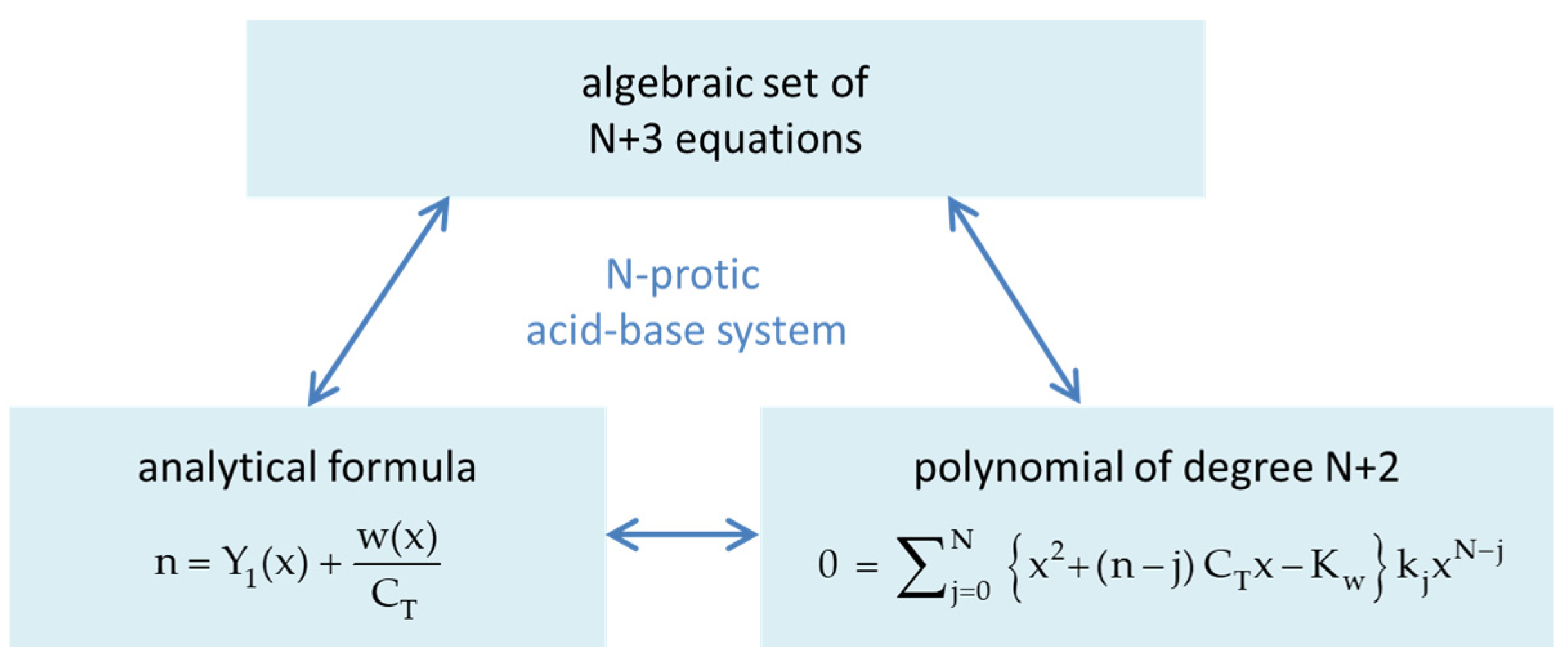



Chemistry Free Full Text Polyprotic Acids And Beyond An Algebraic Approach Html
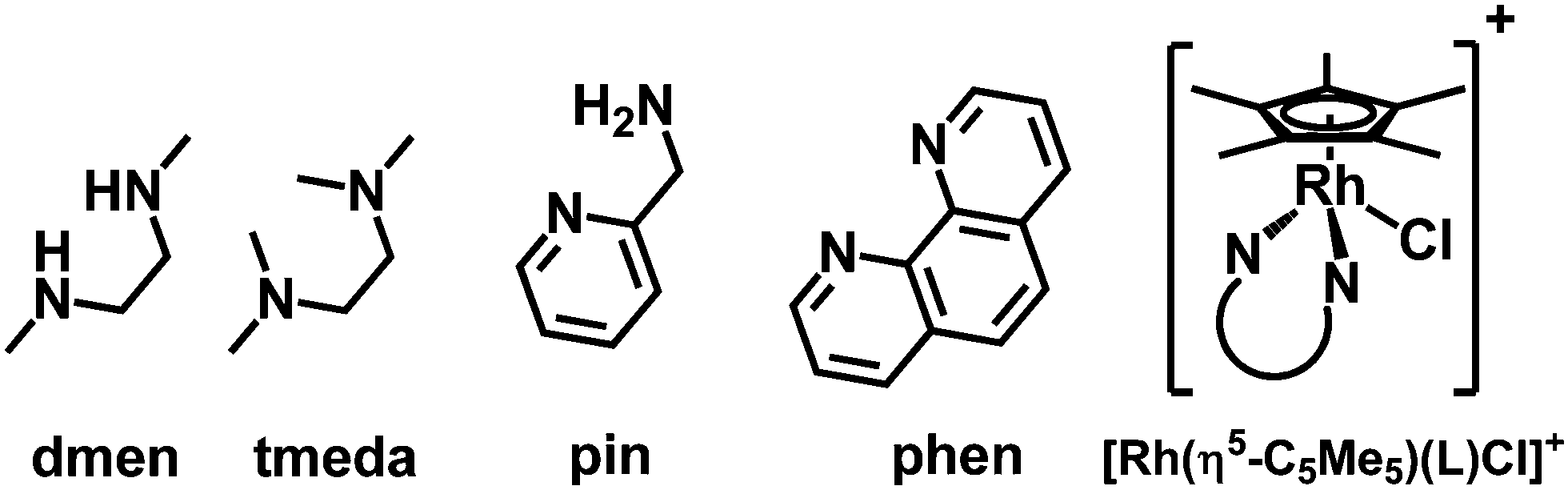



Structural And Solution Equilibrium Studies On Half Sandwich Organorhodium Complexes Of N N Donor Bidentate Ligands New Journal Of Chemistry Rsc Publishing Doi 10 1039 C8njj
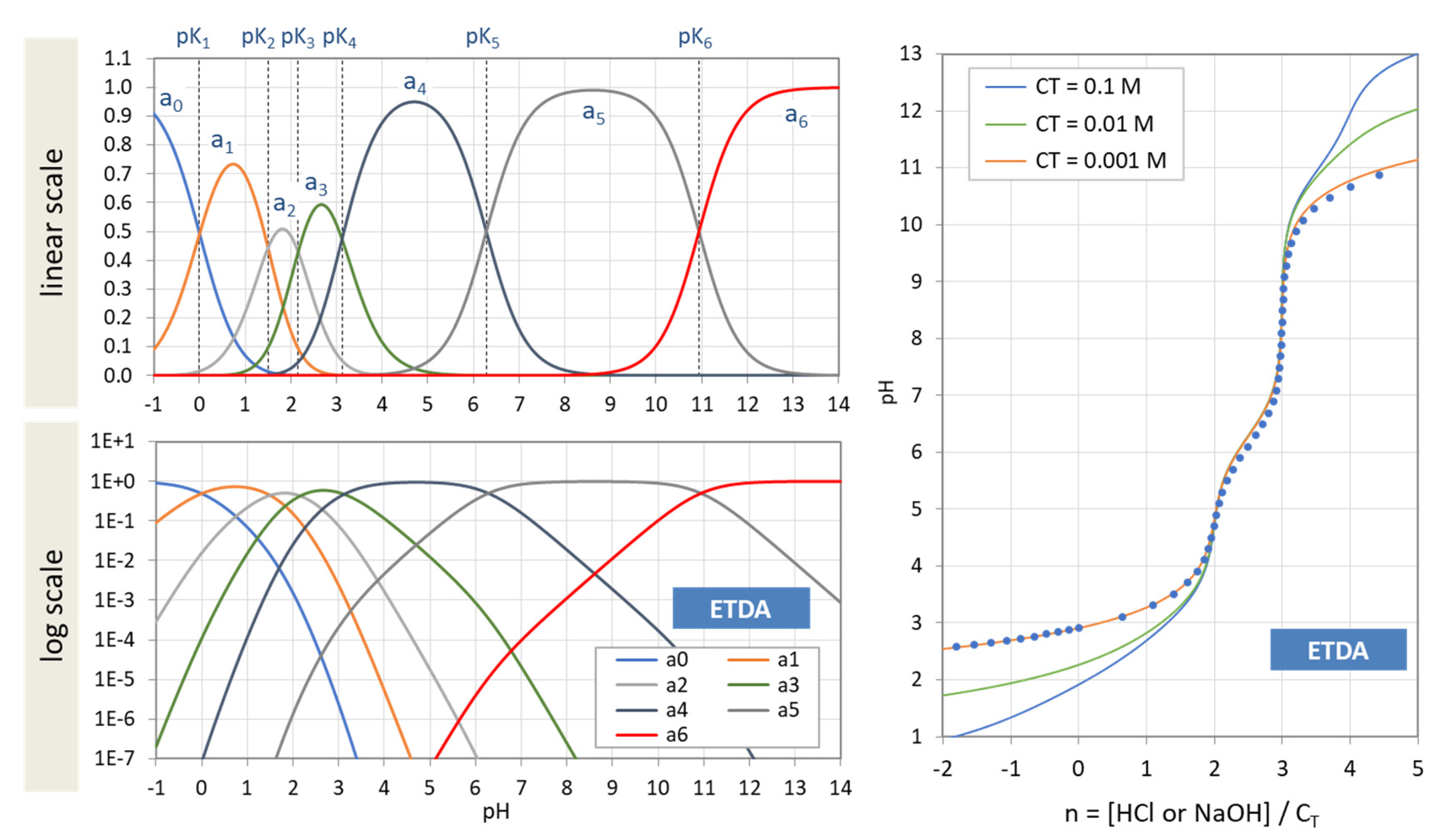



Chemistry Free Full Text Polyprotic Acids And Beyond An Algebraic Approach Html




Mean Chemical Analysis And Formula Unit N 6 Download Table



2




Bio Chem
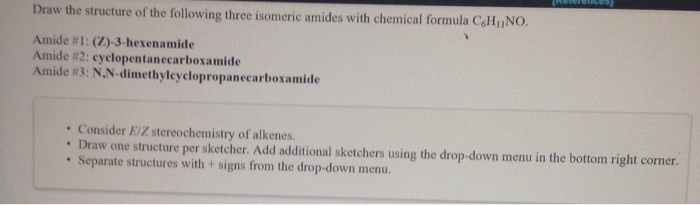



Draw The Structure Of The Following Three Isomeric Chegg Com



2
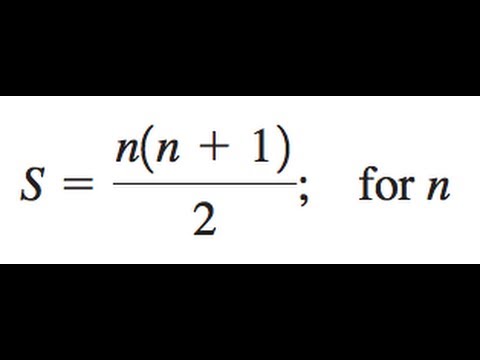



S N N 1 2 Solve For N Youtube




S N N 1 2 Solve For N Youtube



2




Spectroscopic Studies On Degradation Of Poly N N Methylene Bisacrylamide Pdf Free Download



Important Notes Of Chemistry For Neet Jee Structure Of Atom




Chemical Names And Formulas Of Compounds Powerpoint Ppt Download




Chapter 19 Molecules And Compounds 19 1 Chemical




37 How Many Of Each Type Of Atoms Are There In The Formula Nh4c2h302 A N Homeworklib



Unsaturation




Chemical Structure Of Rr 2 Chemical Formula C 19 H 10 Cl 2 N 6 Na 2 O Download Scientific Diagram
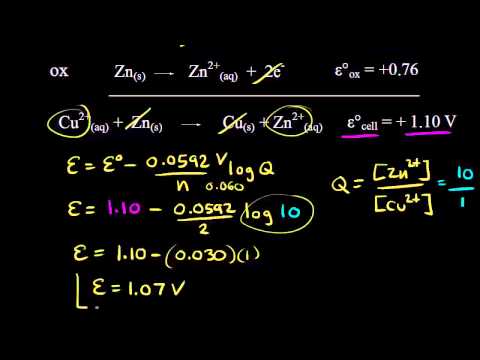



Using The Nernst Equation Video Khan Academy



Q Tbn And9gcsjed7lu7c6mlt5zvgiltbjuofmz Ycvqsesievxze Usqp Cau
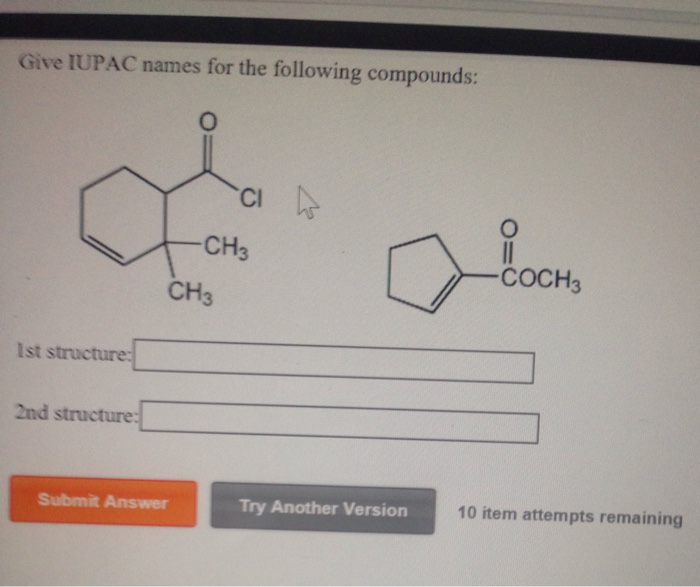



Draw The Structure Of The Following Three Isomeric Chegg Com




4 2 Balancing Chemical Equations 1




The Existence Of Solutions For A Nonlinear Mixed Problem Of Singular Fractional Differential Equations Topic Of Research Paper In Mathematics Download Scholarly Article Pdf And Read For Free On Cyberleninka Open
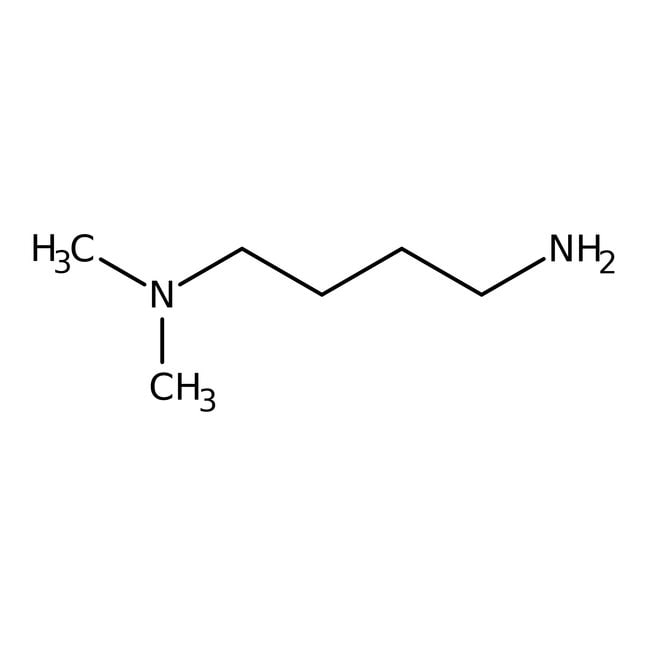



Alfa Aesar N N Dimethyl 1 4 Butanediamine 98 Tertiary Amines Amines Fisher Scientific



On The Theory Of Electron Transfer Reactions Vi Unified Treatment For Homogeneous And Electrode Reactions The Journal Of Chemical Physics Vol 43 No 2



Aip Scitation Org Doi Pdf 10 1063 1




Computational Chemistry Molecular Mechanics Dynamics F Ma Quantum Chemistry Schr O Dinger Equation H E Ppt Download




N 1 Png Images Pngwing
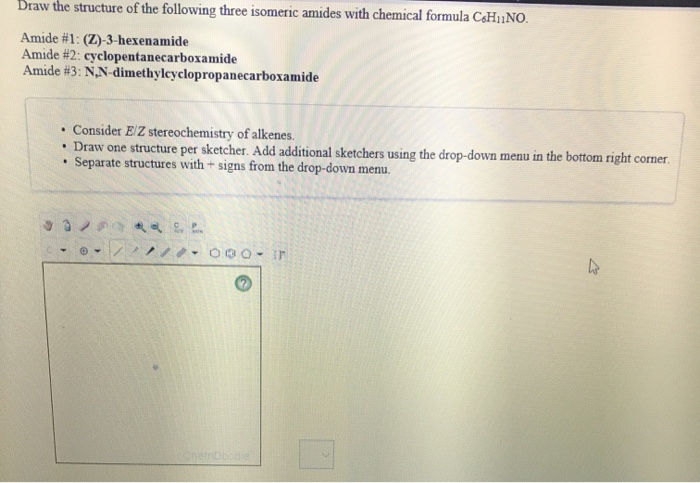



Draw The Structure Of The Following Three Isomeric Chegg Com




N 1 Phenylpropan 2 Yl Hydroxylamine N Hydroxyamph By Asch Issuu
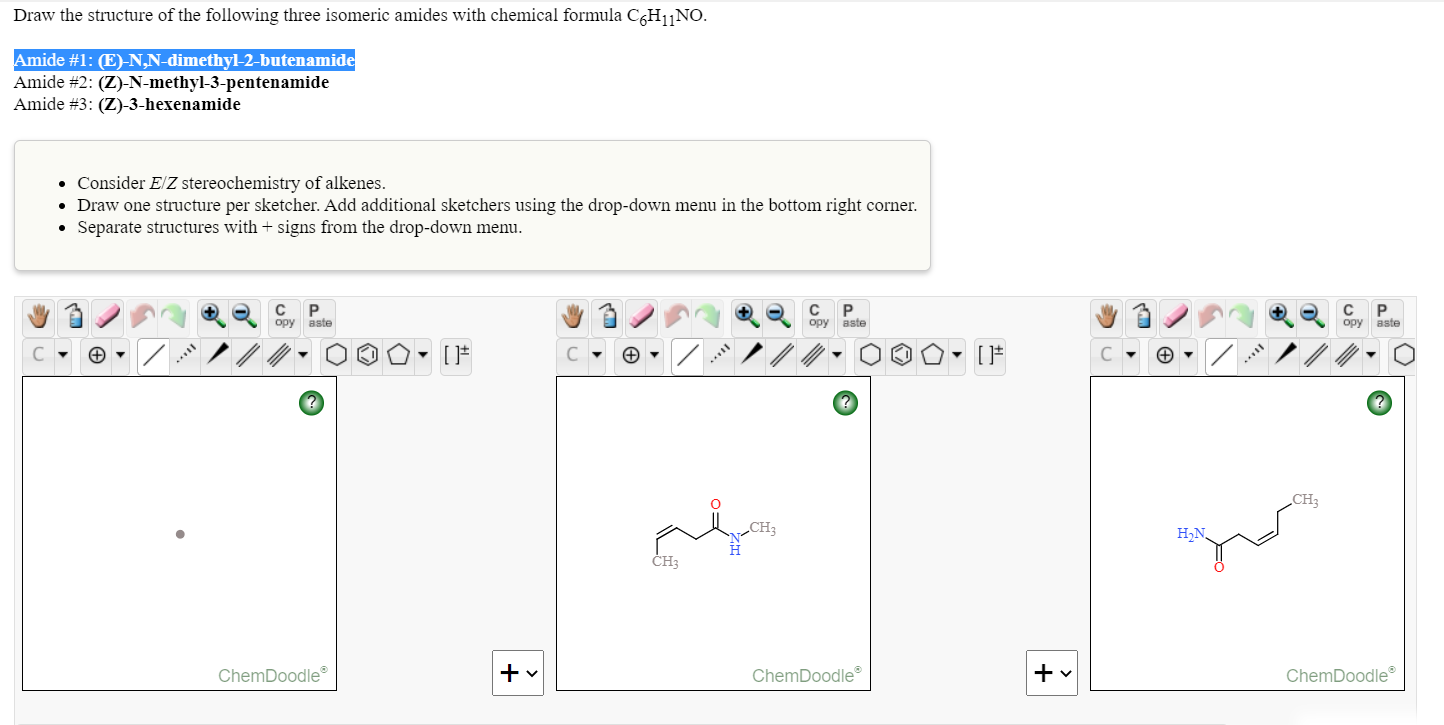



Draw The Structure Of The Following Three Isomeric Chegg Com
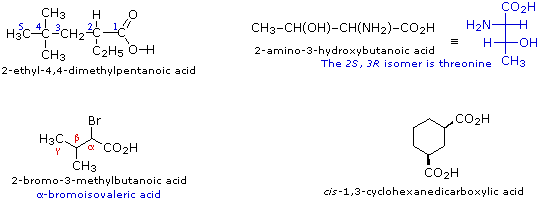



Carboxylic Acid Reactivity




Figure 1 From A Novel Discrete Dinuclear Copper Ii Gadolinium Iii Complex Derived From A Schiff Base Ligand Cu Salbn Gd No3 3 H2o Salbn N N Butylenebis Salicylideaminato Semantic Scholar



0 件のコメント:
コメントを投稿